Rapid H.pylori Ag Test Kit (Colloidal Gold)
- Min. Order:
- 10000 test
- Min. Order:
- 10000 test
- Transportation:
- Ocean, Air, Express, Land
- Port:
- Shenzhen, Guangzhou, Hongkong
Your message must be between 20 to 2000 characters
Contact Now| Place of Origin: | Shenzhen,China |
|---|---|
| Productivity: | 100,000,000,000,000 tests/year |
| Supply Ability: | 100,000,000,000,000 tests/year |
| Payment Type: | L/C,T/T,D/P,D/A,Paypal,Cash |
| Incoterm: | FOB,CFR,CIF,EXW,DDP,Express Delivery |
| Certificate: | CE/SGS |
| HS Code: | 3822009020 |
| Transportation: | Ocean,Air,Express,Land |
| Port: | Shenzhen,Guangzhou,Hongkong |
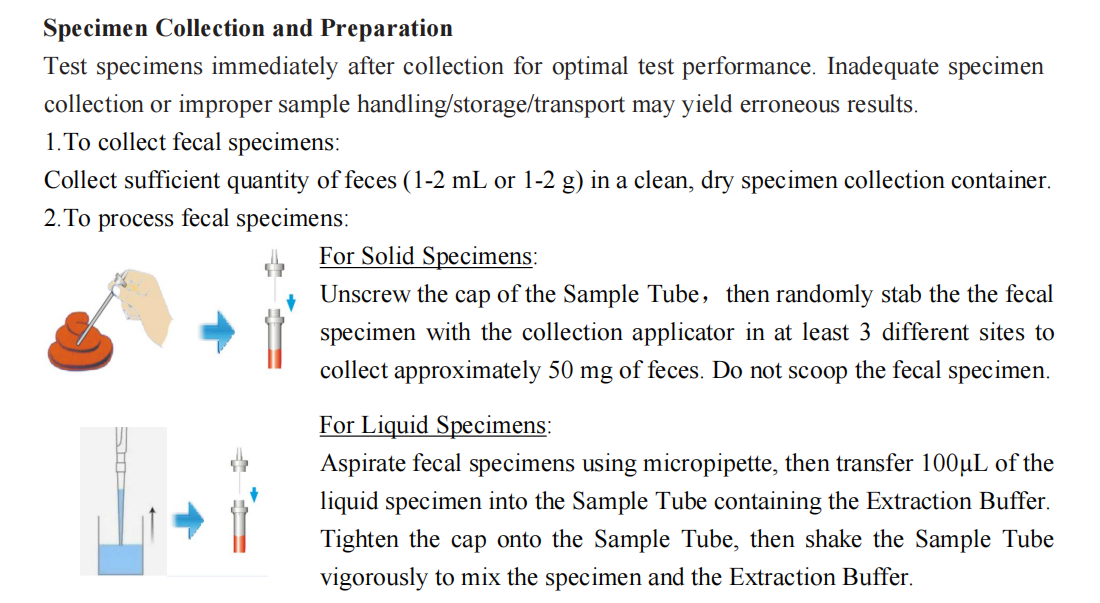
The Rapid H.pylori Ag Test Kit is a lateral flow immunochromatographic assay and intended for qualitative detection of H.pylori antigens in feces specimens to aid in the diagnosis of H. pylori infection.
For professional use only. For in vitro diagnostic use only.
The Rapid H.pylori Ag Test Kit employs a lateral flow chromatographic technology to qualitatively detect the H. pylori antigens in fecal specimens. The fecal sample is collected and treated with the extraction buffer. Add the processed specimen into the test cassette, the specimen will migrate forward along with the test strips through capillary effect. If the H.pylori is present in the specimen, the H.pylori antigens will be captured and detected on the T line, resulting in purplish red band on the test region, indicating a positive result. If the H.pylori is not present or present at very low levels in the specimen, there is no red line appears in T line position. The “Control Line” (C) is used for procedural control. Control line should always appear if the test procedure is performed properly and the test reagents are working.



Related Keywords