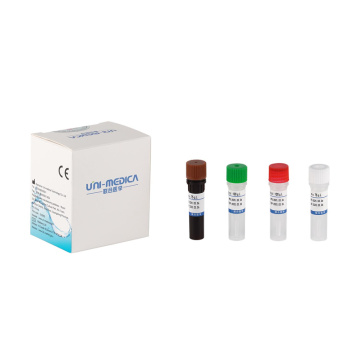
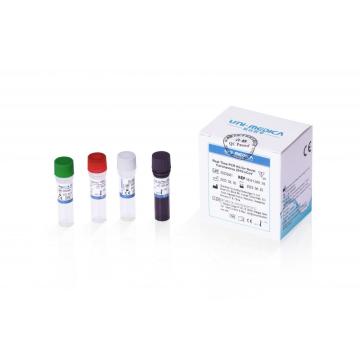
Real-time PCR detection kit for 2019-nCov
- Min. Order:
- 5000 T
- Min. Order:
- 5000 T
- Transportation:
- Ocean, Air, Land
- Port:
- Shenzhen , Guangzhou, Hong Kong
Your message must be between 20 to 2000 characters
Contact Now| Place of Origin: | China |
|---|---|
| Productivity: | 100,000,000T/year |
| Supply Ability: | 10,000,000T/month |
| Payment Type: | L/C,T/T |
| Incoterm: | EXW,FOB,FCA |
| Certificate: | CE, ISO13485, ISO9001 |
| HS Code: | 3822009020 |
| Transportation: | Ocean,Air,Land |
| Port: | Shenzhen ,Guangzhou,Hong Kong |
Due to the Covid-19 pandemic outbreak, Shenzhen Uni-medica has worked extensively to develop the new one step multiplex real-time RT-PCR reagent kit to allow laboratories to diagnose the disease caused by 2019-nCov infection with fast and easy to use assays. Shenzhen Uni-medica RT PCR detection kit is a fast, highly sensitive multiplex diagnostic kit that contains both the assays and controls needed for the real-time PCR detection of RNA from the 2019-nCov.
Shenzhen Uni-medica RT PCR test kit, adopting fluorescence PCR technology, is used for in vitro qualitative DNA detection of novel coronavirus 2019-nCoV in respiratory specimens including oropharyngeal nasopharyngeal swab, sputum, and bronchoalveolar lavage fluid.
Shenzhen Uni-medica RT PCR testing kit has the features below:
High Sensitivity: Limit of detection is 200 copies/ml
Internal Control: The whole process is monitored by internal control;
Rapid Reaction Time: 52 minutes with on a rapid PCR system.
Flexibility: Compatible with multiple brands of Real Time PCR systems.







Related Keywords