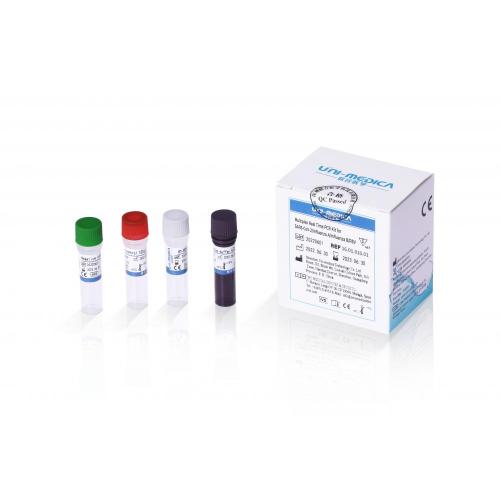
Multiplex Real Time PCR Kit for SARS-CoV-2/Influenza A/Influenza B/RSV
- Min. Order:
- 10000 test
- Min. Order:
- 10000 test
- Transportation:
- Ocean, Land, Air, Express
- Port:
- Shenzhen
Your message must be between 20 to 2000 characters
Contact Now| Place of Origin: | Shenzhen,China |
|---|---|
| Productivity: | 10 000 000 tests/month |
| Supply Ability: | 10 000 000 tests/month |
| Payment Type: | L/C,T/T,D/P,D/A,cash |
| Incoterm: | FOB,CFR,CIF,EXW,FAS,FCA,CPT,DEQ,CIP,DDP,DDU,Express Delivery,DAF,DES |
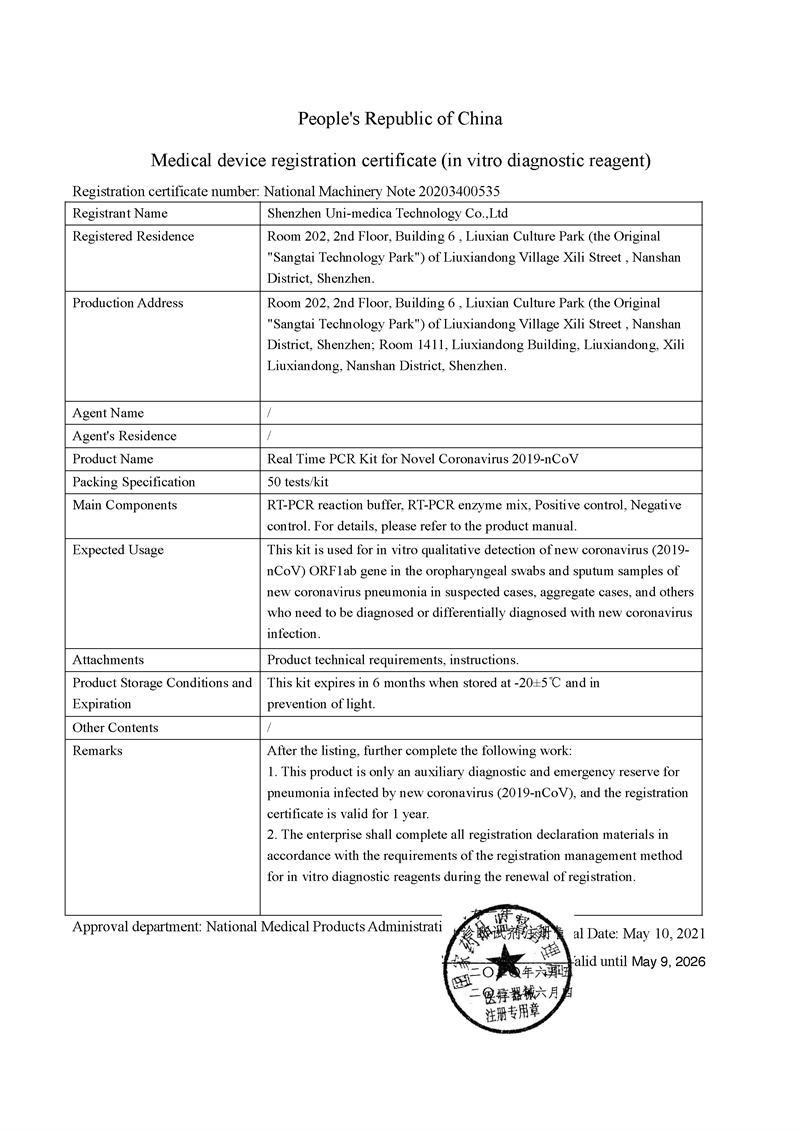
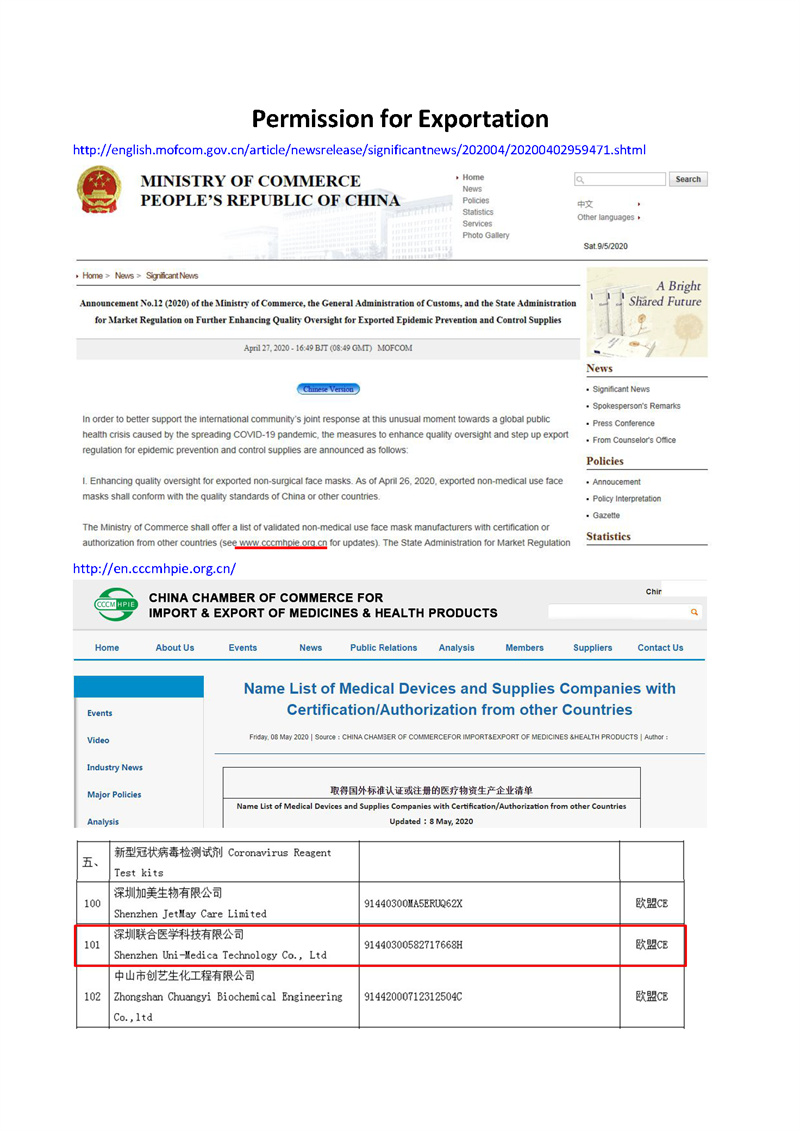
| Certificate: | CE/SGS |
| HS Code: | 3822009020 |
| Transportation: | Ocean,Land,Air,Express |
| Port: | Shenzhen |
Product Name
Multiplex Real Time PCR Kit for SARS-CoV-2/Influenza A/Influenza B/RSV
Alternative Name
Multiplex Real Time PCR Kit for Covid-19+Influenza A/Influenza B+RSV, also named Real Time multiplex PCR test reagent kit for Covid-19+Influenza A/ B+RSV, etc.
Product Description
This kit is used for in vitro qualitative nucleic acid detection of SARS-CoV-2, Influenza A, Influenza B and respiratory syncytial virus (RSV) simultaneously in respiratory specimens including oropharyngeal swabs and sputum, bronchoalveolar lavage fluid and nasopharyngeal swab. Primer sets and FAM labeled probes are
designed for specific detection of both ORFlab and N genes of SARS-CoV-2, ROX labeled probe for M gene of Influenza A, CY5 labeled probe for NS gene of Influenza B, CY5.5 labeled probe for M gene of respiratory syncytial virus (RSV). Human RNase P gene extracted concurrently with the test sample provides an internal control to validate nucleic extraction procedure and reagent integrity. Probe targeting human RNase P gene is labeled with VIC.
Storage and Expiry Date
1. This PCR kit expires in 12 months when stored at -20±5℃ and in prevention of light.
2. Avoid repeated freeze-thaw cycles (5 times maximum).
3. Manufacture date and expiration date are printed on the label.
Applicable instrument
Real-Time PCR System:
Real-Time PCR System: Molarray MA-6000, ABI 7500, ViiATM7, QuantStudio 5, QuantStudio 6/7 pro, QuantStudio 6/7 flex, Agilent Mx3000P/3005P, Rotor-GeneTM6000/Q, Bio-Rad CFX96 TouchTM/iQTM5,Hongshi SLAN-96S/96P,AGS8830, AGS4800, etc.
Specification of RT-PCR reagent kit
Name of product
Multiplex Real Time PCR Kit for SARS-CoV-2+Flu A/B+RSV
Specification
SARS-CoV-2, Flu A,Flu B, RSV
Package
48 tests/kit or 96 tests/kit
Loading port
Shenzhen/Guangzhou/Hongkong
MOQ
9,600 tests
FOB price
negociate
[Our Advantage]
1. High sensitivity: 100-200 copies/ml.
2. Short amplification time: 30-45 mins
3. Quick delivery time: 1-3 days.





Related Keywords