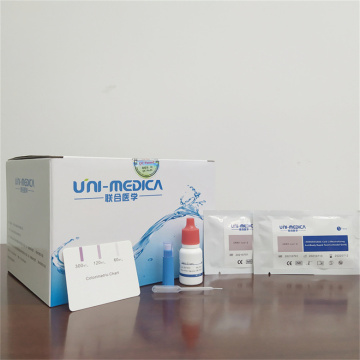
Professional New coronavirus IgM/IgG Duo Rapid Test
- Min. Order:
- 10000 test
- Min. Order:
- 10000 test
- Transportation:
- Ocean, Air, Express, Land
- Port:
- Shenzhen, Guangzhou, Hongkong
Your message must be between 20 to 2000 characters
Contact Now| Place of Origin: | Shenzhen,China |
|---|---|
| Productivity: | 100,000,000,000 tests/year |
| Supply Ability: | 100,000,000,000,000 tests/year |
| Payment Type: | L/C,T/T,D/P,D/A,Paypal,Cash |
| Incoterm: | FOB,CFR,CIF,EXW,DDP,Express Delivery |
| Certificate: | CE/SGS |
| HS Code: | 3822009020 |
| Transportation: | Ocean,Air,Express,Land |
| Port: | Shenzhen,Guangzhou,Hongkong |
Results from the Novel Coronavirus Antibody Test should not be used as the sole basis for diagnosis.
Testing is limited to qualified laboratories. New Coronavirus Antibody Test Results are for the detection of SARS-CoV-2 antibodies. IgM antibodies to SARS-CoV-2 are generally detectable in blood several days after initial infection, although levels over the course of infection are not well characterized. IgG antibodies to SARS-CoV-2 become detectable later following infection.
Positive Sars-cov-2 Antibody Test results for both IgG and IgM could occur after infection and can be indicative of acute or recent infection. Negative 2019-ncov Antibody Test results do not preclude SARS-CoV-2 infection and should not be used as the sole basis for patient management decisions. IgM antibodies may not be detected in the first few days of infection; the sensitivity of the SARS-CoV-2 IgM/IgG Duo Test early after infection is unknown.
False positive results for IgM and IgG antibodies may occur due to cross-reactivity from pre-existing antibodies or other possible causes.At this time, it is unknown for how long IgM or IgG antibodies may persist following infection.
This Antibody Test Kit is for prescription use only and for in vitro diagnostic use only.
Uni-medica always provides high quality and stable quality IVD products to all customers from all over the world.







Related Keywords